Notes to Pathology: Cellular Reaction to Injury
I. ADAPTATION TO ENVIRONMENTAL STRESS
A. Hypertrophy
· Hypertrophy is an increase in the size of an organ or tissue due to an increase in the size of cells.
· Other characteristics include an increase in protein synthesis and an increase in the size or number of intracellular organelles.
· A cellular adaptation to increased workload results in hypertrophy, as exemplified by the increase in skeletal muscle mass associated with exercise and the enlargement of the left ventricle in hypertensive heart disease.
B. Hyperplasia
· Hyperplasia is an increase in the size of an organ or tissue caused by an increase in the number of cells.
· It is exemplified by glandular proliferation in the breast during pregnancy.
· In some cases, hyperplasia occurs together with hypertrophy. During pregnancy, uterine enlargement is caused by both hypertrophy and hyperplasia of the smooth muscle cells in the uterus.
C. Aplasia
· Aplasia is a failure of cell production.
· During fetal development, aplasia results in agenesis, or absence of an organ due to failure of production.
· Later in life, it can be caused by permanent loss of precursor cells in proliferative tissues, such as the bone marrow.
D. Hypoplasia
· Hypoplasia is a decrease in cell production that is less extreme than in aplasia.
· It is seen in the partial lack of growth and maturation of gonadal structures in Turner syndrome and Klinefelter syndrome.
E. Atrophy
· Atrophy is a decrease in the size of an organ or tissue and results from a decrease in the mass of preexisting cells.
· Most often, causal factors are disuse, nutritional or oxygen deprivation, diminished endocrine stimulation, aging, and denervation (lack of nerve stimulation in peripheral muscles caused by injury to motor nerves).
· Characteristic features often include the presence of autophagic granules, which are intracytoplasmic vacuoles containing debris from degraded organelles.
· In some instances, atrophy is thought to be mediated in part by the ubiquitin-proteosome pathway of protein degradation. In this pathway, ubiquitin-linked proteins are degraded within the proteosome, a large cytoplasmic protein complex.
F. Metaplasia is the replacement of one differentiated tissue by another
- Squamous metaplasia
- Squamous metaplasia is exemplified by the replacement of columnar epithelium at the squamocolumnar junction of the cervix by squamous epithelium.
- It can also occur in the respiratory epithelium of the bronchus, in the endometrium, and in the pancreatic ducts.
- Associated conditions include chronic irritation (e.g., squamous metaplasia of the bronchi with long-term use of tobacco) and vitamin A deficiency.
- This process is often reversible.
- Osseous metaplasia
- Osseous metaplasia is the formation of new bone at sites of tissue injury.
- Cartilaginous metaplasia may also occur.
- Myeloid metaplasia (extramedullary hematopoiesis) is proliferation of hematopoietic tissue at sites other than the bone marrow, such as the liver or spleen.
II. HYPOXIC CELL INJURY
A. Causes. Hypoxic cell injury results from cellular anoxia or hypoxia, which in turn results from various mechanisms, including:
- Ischemia (obstruction of arterial blood flow), which is the most common cause
- Anemia, which is a reduction in the number of oxygen-carrying red blood cells
- Carbon monoxide poisoning, which results in diminution in the oxygen-carrying capacity of red blood cells by chemical alteration of hemoglobin
- Decreased perfusion of tissues by oxygen-carrying blood, which occurs in cardiac failure, hypotension, and shock
- Poor oxygenation of blood secondary to pulmonary disease
B. Early stage. Hypoxic cell injury first affects the mitochondria, with resultant decreased oxidative phosphorylation and adenosine triphosphate (ATP) synthesis. Consequences of decreased ATP availability include:
- Failure of the cell membrane pump (ouabain-sensitive Na+-K+-ATPase) results in increased intracellular Na+ and water and decreased intracellular K+. This process causes cellular swelling and swelling of organelles.
- Cellular swelling, or hydropic change, is characterized by the presence of large vacuoles in the cytoplasm.
- Swelling of the endoplasmic reticulum is one of the first ultrastructural changes evident in reversible injury.
- Swelling of the mitochondria progresses from reversible, low-amplitude swelling to irreversible, high-amplitude swelling, which is characterized by marked dilation of the inner mitochondrial space.
- Disaggregation of ribosomes leads to failure of protein synthesis. Ribosomal disaggregation is also promoted by membrane damage.
- Stimulation of phosphofructokinase activity results in increased glycolysis, accumulation of lactate, and decreased intracellular pH. Acidification causes reversible clumping of nuclear chromatin.
C. Late stage
- Hypoxic cell injury eventually results in membrane damage to plasma and to lysosomal and other organelle membranes, with loss of membrane phospholipids.
- Reversible morphologic signs of damage include the formation of:
- Myelin figures, whorl-like structures probably originating from damaged membranes
- Cell blebs, a cell surface deformity most likely caused by disorderly function of the cellular cytoskeleton
D. Cell death. Finally, cell death is caused by severe or prolonged injury.
- The point of no return is marked by irreversible damage to cell membranes, leading to massive calcium influx, extensive calcification of the mitochondria, and cell death.
- Intracellular enzymes and various other proteins are released from necrotic cells into the circulation as a consequence of the loss of integrity of cell membranes. This phenomenon is the basis of a number of useful laboratory determinations as indicators of necrosis.
- Myocardial enzymes in serum.
- Enzymes that have been useful in the diagnosis of myocardial infarction include the following:
- Aspartate aminotransferase (AST, previously known as SGOT)
- Lactate dehydrogenase (LDH)
- Creatine kinase (CK, also known as CPK)
- These markers of myocardial necrosis vary in specificity for heart damage, as well as in the time period after the necrotic event in which elevations in the serum appear and persist. The delineation of isoenzyme forms of LDH and CK has been a useful adjunct in adding specificity to these measures.
- The foregoing enzymes are beginning to be replaced by other myocardial proteins in serum as indicators of myocardial necrosis. Important examples include the troponins (troponin I [TnI] and troponin T [TnT]) and myoglobin.
- Liver enzymes in serum. Enzymes of special interest include the transaminases (AST and alanine aminotransferase [ALT]), alkaline phosphatase, and >-glutamyltransferase (GGT).
- The vulnerability of cells to hypoxic injury varies with the tissue or cell type. Hypoxic injury becomes irreversible after:
- 3–5 minutes for neurons. Purkinje cells of the cerebellum and neurons of the hippocampus are more susceptible to hypoxic injury than are other neurons.
- 1–2 hours for myocardial cells and hepatocytes
- Many hours for skeletal muscle cells
III. FREE RADICAL INJURY
A. Free radicals
- These molecules have a single unpaired electron in the outer orbital.
- Examples include the activated products of oxygen reduction, such as the superoxide (O2-) and the hydroxyl (OH·) radicals.
B. Mechanisms that generate free radicals
- Normal metabolism
- Oxygen toxicity, such as in the alveolar damage that can cause adult respiratory distress syndrome or as in retrolental fibroplasia (retinopathy of prematurity), an ocular disorder of premature infants that leads to blindness
- Ionizing radiation
- Ultraviolet light
- Drugs and chemicals, many of which promote both proliferation of the smooth endoplasmic reticulum (SER) and induction of the P-450 system of mixed function oxidases of the SER. Proliferation and hypertrophy of the SER of the hepatocyte are classic ultrastructural markers of barbiturate intoxication.
- Reperfusion after ischemic injury
C. Mechanisms that degrade free radicals
- Intracellular enzymes, such as glutathione peroxidase, catalase, or superoxide dismutase
- Exogenous and endogenous antioxidants, such as vitamin A, vitamin C, vitamin E, cysteine, glutathione, selenium, ceruloplasmin, or transferrin
- Spontaneous decay
IV. CHEMICAL CELL INJURY
Chemical cell injury is illustrated by the model of liver cell membrane damage induced by carbon tetrachloride (CCl4).
- In this model, CCl4 is processed by the P-450 system of mixed function oxidases within the SER, producing the highly reactive free radical CCl3·.
- CCl3· diffuses throughout the cell, initiating lipid peroxidation of intracellular membranes. Widespread injury results, including:
- Disaggregation of ribosomes, resulting in decreased protein synthesis. Failure of the cell to synthesize the apoprotein moiety of lipoproteins causes an accumulation of intracellular lipids (fatty change).
- Plasma membrane damage, caused by products of lipid peroxidation in the smooth endoplasmic reticulum, resulting in cellular swelling and massive influx of calcium, with resultant mitochondrial damage, denaturation of cell proteins, and cell death
A. General considerations
- Necrosis is one of two contrasting morphologic patterns of tissue death. The other is apoptosis (see VI).
- Necrosis is the sum of the degradative and inflammatory reactions occurring after tissue death caused by injury (e.g., hypoxia, exposure to toxic chemicals); it occurs within living organisms. In pathologic specimens, fixed cells with well-preserved morphology are dead but not necrotic.
- Autolysis refers to degradative reactions in cells caused by intracellular enzymes indigenous to the cell. Postmortem autolysis occurs after the death of the entire organism and is not necrosis.
- Heterolysis refers to cellular degradation by enzymes derived from sources extrinsic to the cell (e.g., bacteria, leukocytes).
B. Types of necrosis
- Coagulative necrosis
- Coagulative necrosis results most often from a sudden cutoff of blood supply to an organ (ischemia), particularly the heart and kidney.
- General preservation of tissue architecture is characteristic in the early stages.
- Increased cytoplasmic eosinophilia occurs because of protein denaturation and loss of cytoplasmic RNA.
- Nuclear changes, the morphologic hallmark of irreversible cell injury and necrosis, are characteristic. These include:
- Pyknosis, chromatin clumping and shrinking with increased basophilia
- Karyorrhexis, fragmentation of chromatin
- Karyolysis, fading of chromatin material
- Disappearance of stainable nuclei
- Liquefactive necrosis
- Ischemic injury to the central nervous system (CNS) characteristically results in liquefactive necrosis. After the death of CNS cells, liquefaction is caused by autolysis.
- Digestion, softening, and liquefaction of tissue are characteristic.
- Suppurative infections characterized by the formation of pus (liquefied tissue debris and neutrophils) by heterolytic mechanisms involve liquefactive necrosis.
- Caseous necrosis
- This type of necrosis occurs as part of granulomatous inflammation and is a manifestation of partial immunity caused by the interaction of T lymphocytes (CD4+, CD8+, and CD4-CD8-), macrophages, and probably cytokines, such as interferon-γ, derived from these cells.
- Tuberculosis is the leading cause of caseous necrosis.
- Caseous necrosis combines features of both coagulative necrosis and liquefactive necrosis.
- On gross examination, caseous necrosis has a cheese-like (caseous) consistency.
- On histologic examination, caseous necrosis has an amorphous eosinophilic appearance.
- Gangrenous necrosis
- This type of necrosis most often affects the lower extremities or bowel and is secondary to vascular occlusion.
- When complicated by infective heterolysis and consequent liquefactive necrosis, gangrenous necrosis is called wet gangrene.
- When characterized primarily by coagulative necrosis without liquefaction, gangrenous necrosis is called dry gangrene.
- Fibrinoid necrosis
- This deposition of fibrin-like proteinaceous material in the arterial walls appears smudgy and acidophilic.
- Fibrinoid necrosis is often associated with immune-mediated vascular damage.
- Fat necrosis occurs in two forms.
- Traumatic fat necrosis, which occurs after a severe injury to tissue with high fat content, such as the breast
- Enzymatic fat necrosis, which is a complication of acute hemorrhagic pancreatitis, a severe inflammatory disorder of the pancreas
- Proteolytic and lipolytic pancreatic enzymes diffuse into inflamed tissue and literally digest the parenchyma.
- Fatty acids liberated by the digestion of fat form calcium salts (saponification, or soap formation).
- Vessels are eroded, with resultant hemorrhage.
A. General considerations
- Apoptosis is a second morphologic pattern of tissue death. (The other is necrosis; see V.) It is often referred to as programmed cell death.
- This is an important mechanism for the removal of cells. An example is apoptotic removal of cells with irreparable DNA damage (from free radicals, viruses, cytotoxic immune mechanisms), protecting against neoplastic transformation
- In addition, apoptosis is an important mechanism for physiologic cell removal during embryogenesis and in programmed cell cycling (e.g., endometrial cells during menstruation).
- This involutional process is similar to the physiologic loss of leaves from a tree; apoptosis is a Greek term for "falling away from."
B. Morphologic features
- A tendency to involve single isolated cells or small clusters of cells within a tissue
- Progression through a series of changes marked by a lack of inflammatory response
- Blebbing of plasma membrane, cytoplasmic shrinkage, chromatin condensation
- Budding of cell and separation of apoptotic bodies (membrane-bound segments)
- Phagocytosis of apoptotic bodies
- Involution and shrinkage of affected cells and cell fragments, resulting in small round eosinophilic masses often containing chromatin remnants, exemplified by Councilman bodies in viral hepatitis
C. Biochemical events
- Diverse injurious stimuli (e.g., free radicals, radiation, toxic substances, withdrawal of growth factors or hormones) trigger a variety of stimuli, including cell surface receptors such as FAS, mitochondrial response to stress, and cytotoxic T cells.
- The extrinsic pathway of initiation is mediated by cell surface receptors exemplified by FAS, a member of the tumor necrosis factor receptor family of proteins. This pathway is initiated by signaling by molecules such as the FAS ligand, which in turn signals a series of events that involve activation of caspases. Caspases are aspartate-specific cysteine proteases that have been referred to as "major executioners" or "molecular guillotines." The death signals are conveyed in a proteolytic cascade, through activation of a chain of caspases and other targets. The initial activating caspases are caspase-8 and caspase-9, and the terminal caspases (executioners) include caspase-3 and caspase-6 (among other proteases).
- The intrinsic, or mitochondrial, pathway, which is initiated by the loss of stimulation by growth factors and other adverse stimuli, results in the inactivation and loss of bcl-2 and other antiapoptotic proteins from the inner mitochondrial membrane. This loss results in increased mitochondrial permeability, the release of cytochrome c, and the stimulation of proapoptotic proteins such as bax and bak. Cytochrome c interacts with Apaf-1 causing self-cleavage and activation of caspase-9. Downstream caspases are activated by upstream proteases and act themselves to cleave cellular targets.
- Cytotoxic T-cell activation is characterized by direct activation of caspases by granzyme B, a cytotoxic T-cell protease that perhaps directly activates the caspase cascade. The entry of granzyme B into target cells is mediated by perforin, a cytotoxic T-cell protein.
- Degradation of DNA by endonucleases into nucleosomal chromatin fragments that are multiples of 180–200 base pairs results in the typical "laddering" appearance of DNA on electrophoresis. This phenomenon is characteristic of, but not entirely specific for, apoptosis.
- Activation of transglutaminases crosslinks apoptotic cytoplasmic proteins.
- The caspases consist of a group of aspartic acid-specific cysteine proteases that are activated during apoptosis.
- Newer methods such as the TUNEL assay (Terminal Transferase dUTP Nick End Labeling) are ways to quantitate cleaving of nucleosomes and, thus, apoptosis. Similarly, caspase assays are coming into use as apoptotic markers. Surely more will follow.
D. Regulation of apoptosis is mediated by a number of genes and their products. Important genes include bcl-2 (gene product inhibits apoptosis), bax (gene product facilitates apoptosis), and p53 (gene product decreases transcription of bcl-2 and increases transcription of bax, thus facilitating apoptosis).
E. Additionally, complex signaling pathways involving multiple genes and gene products are the subject of vigorous scientific investigation. Since many pathologic processes are related to either stimulation or inhibition of apoptosis (e.g., many forms of cancer), this area of inquiry promises to yield major understanding that will surely lead to important therapeutic applications.
 |
| Hereditary hemochromatosis. Prussian blue staining marks the intraparenchymal deposition of hemosiderin. |
VII. REVERSIBLE CELLULAR CHANGES AND ACCUMULATIONS
A. Fatty change (fatty metamorphosis, steatosis)
- General considerations
- Fatty change is characterized by the accumulation of intracellular parenchymal triglycerides and is observed most frequently in the liver, heart, and kidney. For example, in the liver, fatty change may be secondary to alcoholism, diabetes mellitus, malnutrition, obesity, or poisonings.
- Imbalance among the uptake, utilization, and secretion of fat is the cause of fatty change, and this can result from any of the following mechanisms:
- Increased transport of triglycerides or fatty acids to affected cells
- Decreased mobilization of fat from cells, most often mediated by decreased production of apoproteins required for fat transport. Fatty change is thus linked to the disaggregation of ribosomes and consequent decreased protein synthesis caused by failure of ATP production in CCl4-injured cells.
- Decreased use of fat by cells
- Overproduction of fat in cells
B. Hyaline change
- This term denotes a characteristic (homogeneous, glassy, eosinophilic) appearance in hematoxylin and eosin sections.
- It is caused most often by nonspecific accumulations of proteinaceous material.
C. Accumulations of exogenous pigments
- Pulmonary accumulations of carbon (anthracotic pigment), silica, and iron dust
- Plumbism (lead poisoning)
- Argyria (silver poisoning), which may cause a permanent gray discoloration of the skin and conjunctivae
D. Accumulations of endogenous pigments
- Melanin
- This pigment is formed from tyrosine by the action of tyrosinase, synthesized in melanosomes of melanocytes within the epidermis, and transferred by melanocytes to adjacent clusters of keratinocytes and also to macrophages (melanophores) in the subjacent dermis.
- Increased melanin pigmentation is associated with suntanning and with a wide variety of disease conditions.
- Decreased melanin pigmentation is observed in albinism and vitiligo.
- Bilirubin
- This pigment is a catabolic product of the heme moiety of hemoglobin and, to a minor extent, myoglobin.
- In various pathologic conditions, bilirubin accumulates and stains the blood, sclerae, mucosae, and internal organs, producing a yellowish discoloration called jaundice.
- Hemolytic jaundice, which is associated with the destruction of red cells.
- Hepatocellular jaundice, which is associated with parenchymal liver damage, and obstructive jaundice, which is associated with intra- or extrahepatic obstruction of the biliary tract.
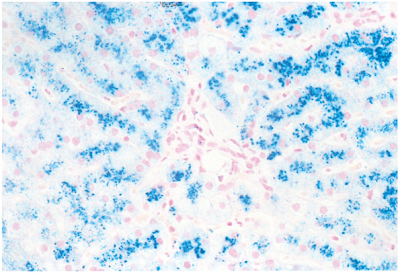
- Hemosiderin
- This iron-containing pigment consists of aggregates of ferritin. It appears in tissues as golden brown amorphous aggregates and can be positively identified by its staining reaction (blue color) with Prussian blue dye. It exists normally in small amounts as physiologic iron stores within tissue macrophages of the bone marrow, liver, and spleen.
- It accumulates pathologically in tissues in excess amounts (sometimes massive).
- Hemosiderosis is defined by accumulation of hemosiderin, primarily within tissue macrophages, without associated tissue or organ damage.
- Hemochromatosis is more extensive accumulation of hemosiderin, often within parenchymal cells, with accompanying tissue damage, scarring, and organ dysfunction. This condition occurs in both hereditary (primary) and secondary forms.
- Hereditary hemochromatosis is most often caused by a mutation in the Hfe gene on chromosome 6.
- Hemosiderin deposition and organ damage in the liver, pancreas, myocardium, and multiple endocrine glands is characteristic, as well as melanin deposition in the skin.
- This results in the triad of micronodular cirrhosis, diabetes mellitus,skin pigmentation. This set of findings is referred to as "bronze diabetes." Laboratory abnormalities of note include marked elevation of the serum transferrin saturation because of the combination of increased serum iron and decreased total iron-binding capacity (TIBC). and
- Secondary hemochromatosis is most often caused by multiple blood transfusions administered to subjects with hereditary hemolytic anemias such as β-thalassemia major (Figure 1-4).
- Lipofuscin
- This yellowish, fat-soluble pigment is an end product of membrane lipid peroxidation.
- It is sometimes referred to as "wear-and-tear" pigment.
- It commonly accumulates in elderly patients, in whom the pigment is found most often within hepatocytes and at the poles of nuclei of myocardial cells. The combination of lipofuscin accumulation and atrophy of organs is referred to as brown atrophy.
E. Pathologic calcifications
- Metastatic calcification
- The cause of metastatic calcification is hypercalcemia.
- Hypercalcemia most often results from any of the following causes:
- Hyperparathyroidism
- Osteolytic tumors with resultant mobilization of calcium and phosphorus
- Hypervitaminosis D
- Excess calcium intake, such as in the milk-alkali syndrome (nephrocalcinosis and renal stones caused by milk and antacid self-therapy)
- Dystrophic calcification
- Dystrophic calcification is defined as calcification in previously damaged tissue, such as areas of old trauma, tuberculosis lesions, scarred heart valves, and atherosclerotic lesions.
- The cause is not hypercalcemia; typically, the serum calcium concentration is normal (Figure 1-5).
VIII. DISORDERS CHARACTERIZED BY ABNORMALITIES OF PROTEIN FOLDING
A. These disorders involve failure of protein structural stabilization or degradation by specialized proteins known as chaperones. Important chaperones include heat shock proteins induced by stress, one of which is ubiquitin, which marks abnormal proteins for degradation.
B. Two known pathogenetic mechanisms include:
- Abnormal protein aggregation, which is characteristic of amyloidosis; a number of neurodegenerative diseases, such as Alzheimer disease, Huntington disease, and Parkinson disease; and perhaps prion diseases, such as "mad cow" disease
- Abnormal protein transport and secretion, which is characteristic of cystic fibrosis and α1-antitrypsin deficiency



